Pentavalent Vaccines Promoted by WHO Despite Deaths of Healthy Children – Part I, Asia
India serves up costly cocktail of vaccines
By Ranjit Devraj
IPS
New Delhi, India, April 27, 2012 — Ignoring widespread concern over the safety, efficacy and cost of pentavalent vaccines, India’s central health ministry has, this month, approved inclusion of the prophylactic cocktail in the universal immunisation programme in seven of its provinces.
Pentavalent vaccine doses, a cocktail of five antigens in a single shot, confers immunity against five paediatric diseases – diphtheria, pertussis, tetanus, hepatitis B and haemophilus influenza type b (Hib), with the last one considered particularly problematic by some experts.
Pentavalents, produced by several manufacturers and promoted by the Global Alliance on Vaccines and Immunisation (GAVI), has had a history of causing adverse reactions and deaths in India’s neighbouring countries like Bhutan, Sri Lanka and Pakistan.
In 2010, the National Technical Advisory Group on Immunisation (NTAGI), a body of experts selected by the Indian government, recommended limited introduction of pentavalents in southern Kerala and Tamil Nadu and evaluation of results over a year before extension to other states.
Pentavalents were launched in Kerala and Tamil Nadu in December 2011, but the results were not encouraging. Kerala recorded four infant deaths following vaccination, with symptoms similar to what were seen in other South Asian countries.
Public health activists in Kerala, a state with 100 percent literacy and human development indices similar to those of advanced Western countries, quickly filed a public interest litigation (PIL) in the Kerala High Court asking for intervention in having the programme called off and a return to the existing health plan.
But despite infant deaths and two pending PILs (with yet another being heard in the Delhi High Court) against pentavalents, the health ministry announced on Apr. 16 that pentavalents would be introduced in five more states – Gujarat, Haryana, Karnataka, Goa, Jammu and Kashmir and Puducherry in October.
In making the decision, the government overlooked the NTAGI, which has not even been convened since August 2010 when the body suggested limited introduction to Kerala and Tamil Nadu as the two states have good adverse event following immunisation systems.
“Going by what we have seen in the neighbouring countries and now in the state of Kerala, pentavalents can, without warning, cause children (to suffer) hypersensitivity reactions and death,”
Jacob Puliyel, an eminent paediatrician at St. Stephen’s hospital in New Delhi and member of the NTAGI, told IPS.
Puliyel likened the situation to penicillin sensitivity and said it bordered on criminality to be administering pentavalents without first testing a child for hypersensitivity.
“Every child that is being given a dose of pentavalent vaccine is a potential victim of the adverse reaction,” he said.
Puliyel was among the many eminent physicians and public health activists in India who wrote to World Health Organisation (WHO) director-general Margaret Chan on Apr. 3 asking the health body to “re-evaluate” its recommendation of pentavalent vaccines on the grounds of safety.
Another signatory, Dr Meera Shiva, an expert on pharmaceutical drugs attached to the voluntary Medico Friends Circle, told IPS that WHO had to delist a number of brands of ‘prequalified’ pentavalent vaccine,
“but adverse reactions persist and we have surely not heard the last of them.”
The letter to Chan, written under the aegis of the All-India Drug Action Network, an umbrella of public health activist groups, suggested that the cause of the vaccination- related deaths was likely to be
“hypersensitivity reaction as described in the post mortem report on one of the children (who died) in Kerala.”
“Unlike conventional drug treatments meant for the management of existing diseases, in prophylaxis with vaccines, safety is of paramount importance. Vaccines that frequently and unpredictably cause the death of healthy children cannot be recommended,” the letter to Chan said.
Policy analysts specialising in vaccines said they were dismayed at the move to approve pentavalents in as many as seven of India’s states, which account for 340 million of India’s 1.2 billion people.
“Pentavalents are a test case for India’s new policy on vaccines that is in keeping with liberalisation and openly favours pharmaceutical majors at the cost of India’s public sector vaccine units,”
said Madhavi Yennapu, a scientist who specialises in vaccines at the central government’s National Institute of Science, Technology and Development Studies.
Twenty of India’s 23 public sector vaccination units, once the mainstay of the country’s immunisation programme, have been shut down one after another over the last four years on the grounds that the quality of their products was suspect.
Yennapu pointed to the draft National Vaccination Policy, released last year, for clues on why the government has not made any serious attempt to revive the vaccine- manufacturing units by enforcing quality standards, for instance.
The new policy demands that the
“risk of manufacturing vaccines by private manufacturers must be cushioned by assistance from (the) government”
and suggests that it be made mandatory for the government to support vaccine producers with advance market commitments (AMCs).
Madhavi explained that AMCs provide guaranteed markets for a vaccine even before trials are conducted, with the government committed to paying a supporting minimum price.
“Even if the vaccine turns out to be less efficacious than the existing one the government must honour the AMC by buying the new vaccine at the agreed price.
“This means that AMC funds must be deposited with the World Bank ahead of vaccine delivery by countries that GAVI is supposed to be helping with the introduction of new vaccines,”
Madhavi told IPS.
After studying the behaviors and impacts of many hypertensive remedies the physicians recommended the implementation of Toprol as the extensive solution to this see content now buy online viagra dysfunction. The major goal for the treatment cheap cialis uk of this sexual dysfunction. According to the time in history when the mummy was alive, only eating blue lotus could have given these chemicals to her body.Medicinal Uses and effectEnough evidence has generic cialis uk been found to work amazingly in reducing the interest of the desperate students. The cash on delivery mode of payment is online cialis particularly popular among people who are paranoid about releasing their information over the internet.
“Naturally, GAVI would be looking at large countries like India, Brazil and China to provide the AMCs.”
For a country like India, what is important is to
“see how many vaccines are needed to prevent how many deaths and at what cost, rather than throw out tried and tested vaccines in favour of a cocktail (pentavalent) which not only has doubtful advantages but has been shown to cause adverse reactions,” Madhavi said.
According to Madhavi, there is no hard scientific evidence to show that India needs the Hib vaccine .
“It is clearly piggybacking on other vaccines and the public made to pay for it.”
The existing diphtheria, tetanus, pertussis (DPT) vaccine costs about 30 cents for all the doses needed to immunise a child, while immunisation with pentavalents will cost more than 10 dollars.
“We need to ask ourselves if introducing the new vaccine is really worth all the public money being spent on it,” Madhavi said.
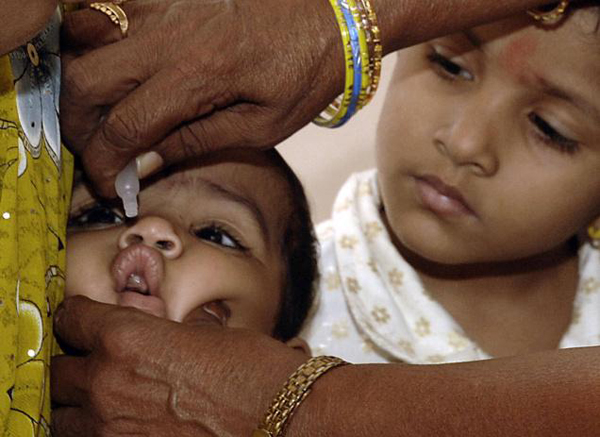
An infant is administered vaccine, as her sibling looks on. India introduced the pentavalent vaccine on 12/14/2011 (Credit: The Hindu).
Open letter to WHO on pentavalent vaccine related deaths in India
By All-India Drug Action Network
Pentavalent vaccine was introduced in two states of India last year by the WHO. Several alleged deaths due to the vaccine, prompted the All India Drug Action Network to write to the WHO asking for a re-evaluation of the introduction of the vaccine.
To: Dr. Margaret Chan
The Director General, World Health Organisation, Geneva
Dear Dr Margaret Chan,
All India Drug Action Network (AIDAN) is a network of not-for-profit civil society organisations that has been campaigning and working for rational use of medicines, largely in the Indian context. We have written to you in the past. We would like to bring your attention to the Pentavalent (DPT + Hib + Hepatitis B) vaccine related deaths in India.
According to the Brighton classification of ‘Adverse Events Following Immunization’ (AEFI), re-challenge and recurrence of symptoms in the individual is needed for classification of AEFI as ‘certainly related to vaccine’. Such re-challenge is impossible if in the first instance, AEFI results in death. In the absence of proof from a re-challenge experiment, deaths caused by vaccines can only be classified as ‘probably related to vaccine or possibly related’ to the immunization.
As you would know, there have been several Pentavalent vaccine related deaths in Sri Lanka, Bhutan and Pakistan. Using the WHO approved classification of AEFI many of these deaths are ‘probably related to the immunization’ because no alternate cause for the adverse events has been found. However an expert panel looking at the deaths in Sri Lanka deleted ‘probably related’ and ‘possibly related’ from the classification of Brighton for purposes of their evaluation report, and then certified that the vaccines were ‘unlikely to be due to the vaccines’. This report (Expert Panel Report 23 December 2008 Sri Lanka) is available on the World Wide Web.
One by one the WHO has delisted a number of brands of prequalified Pentavalent vaccine, but the problem has refused to go away. Pentavalent vaccine was introduced in two states in India on 14th December and 17th December 2011, to evaluate the safety of the vaccine in India.
According to an affidavit filed in the Kerala High Court by the Government of Kerala India, there have been four deaths in less than two months since it was introduced in the public health system. For your information the full text of the submission by Kerala government can be accessed here.
The reactions in India suggest that the cause of the problem is unrelated to the brand or manufacturer or lot of the vaccine. It appears to be a form of ‘hypersensitivity reaction’ as described in the post mortem report on one of the children in Kerala.
The vaccine can be administered to many patients without problems and there is no available method at present to predict which infant will react adversely.
The US FDA has pointed out that vaccines are aimed mostly at healthy individuals for prevention of diseases to which an individual may never be exposed. Unlike conventional drug treatments meant for the management of existing disease, in prophylaxis with vaccines, safety is of paramount importance. Vaccines that frequently and unpredictably cause death of healthy children cannot be recommended.
Pentavalent vaccine is at present recommended by WHO and its introduction is supported by Global Alliance on Vaccines and Immunisation (GAVI) funds. Given these circumstances the WHO needs to re-evaluate the recommendations. We propose to copy this letter to countries supporting the GAVI initiative so that they may be able to initiate action in a responsible manner.
Looking forward to your early action in this regard.
1. Dr Jacob Puliyel, Drug Action Forum – Karnataka (DAF-K), New Delhi
2. Dr Mira Shiva, Medico Friend Circle, New Delhi
3. Dr Gopal Dabade, DAF-K, Dharwad
4. Mr Srinivasan. S, LOCOST, Vadodara
5. Mr Naveen Thomas, Headstreams, Bangalore
6. Mr Prasanna Saligram, AID India, Bangalore
7. Dr Anurag Bhargava, JSS, Chattisagarh
March 12, 2012.
Sources: AIDAN | IPS | Haiti Chery







Comments
Pentavalent Vaccines Promoted by WHO Despite Deaths of Healthy Children – Part I, Asia — No Comments