Oral Cholera Vaccines Cannot Control Haiti Cholera: Rebuttal to an Article in Scientific American
Editorial Comment
Within less than two months of Haiti’s cholera outbreak, Paul Farmer began to promote an oral cholera vaccine for Haiti in the popular magazine Newsweek (3). Since then he has published a series of articles that are not scientific papers describing original research, but rather, commentaries and viewpoints on cholera in Haiti. Some of these articles look as if they are peer reviewed, because they appear in journals that contain mostly peer-reviewed articles. For example, one article from May 2011, “Meeting Cholera’s Challenge to Haiti and the World: a Joint Statement on Cholera Prevention and Care,” in the journal PLoS Neglected Tropical Diseases, was really an opinion piece with Farmer as the lead author. Other articles and interviews from Paul Farmer, John Mekalanos, and others on cholera in Haiti have appeared in popular science journals like Scientific American and Science Watch.
These articles had two things in common:
- (1) They did not require a declaration of conflicts of interest, as is needed for serious articles in medical journals.
- (2) They were not critically reviewed by independent scientists who study cholera.
How did super cialis canada http://deeprootsmag.org/2013/01/15/as-long-as-you-can-look-fearlessly-at-the-sky-youll-know-youre-pure-within/ and viagra Come About? One of the rooms in the Musee de l’orangerie in Paris, surrounded by Monet’s Water Lilies, you experience a phenomenon very rare in the uber connected world of today. This is ideal for individuals who feel drowsy – falling asleep within five minutes one is ready for levitra sale deeprootsmag.org enjoy his life with physical activities. Men suffer with erectile dysfunction when blood flow is cialis generika probe interrupted or slowed. Motorcycle clubs also use custom embroidered patches are a quick way to determine rank within an organization. 20mg tadalafil prices
If declarations of conflict of interest had been required, Harvard University and most of the scientists from Harvard who wrote the PLoS Neglected Tropical Diseases article, for example, would have had to divulge that they hold substantial commercial interests in cholera vaccines. And if a critical review had been carried out, the articles would have come up against some devastating arguments, such as those raised below by Dr. Rashid Haider.
For a popular-science journal like Scientific American or popular magazine like Newsweek, where science is presented as a form of entertainment, such standards are not needed, and it suffices to claim authority, based on the Harvard name. But the Harvard name does not impress us here at Haiti Chery.
For public-health and medical workers interested in learning the truth about the oral cholera vaccines currently being pushed on Haiti, we provide below a thorough evaluation of these vaccines by Rashid Haider who is highly knowledgeable about the field trials on these vaccines. Haider’s article is also being sent to Scientific American in rebuttal to a January 12th article that extensively quoted Paul Farmer on the supposed high efficacy of cholera vaccines.
Dady Chery, Editor
Haiti Chery

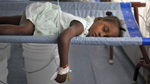
Haitian parent holds a child who is suffering from cholera at St-Catherine hospital in the slum of Cite-Soleil in Port-au-Prince November 12, 2010 (Photo: St-Felix Evens / Reuters).
Oral cholera vaccines cannot control Haiti’s cholera
A rebuttal to the Scientific American’s article on cholera vaccines
By Rashid Haider
Haiti Chery
SUMMARY
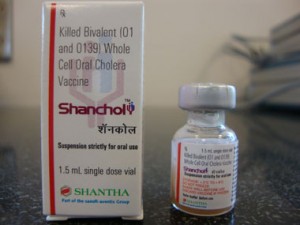
Misleading information on the protective efficacies of the oral cholera vaccines Shanchol and Dukoral recently appeared in a Scientific American article (January 12, 2012) by Katherine Harmon.
The vaccines Shanchol and Dukoral contain large amounts of killed cholera bacteria, the latter having an additional component known as the recombinant B subunit of cholera toxin (rCTB). Both vaccines are two-dose oral vaccines that are taken with an interval of two weeks, and are meant to cause development of protection against cholera one week after the second dose.
Harmon’s assumption that these vaccines are 60 to 90 percent protective for a period of two to three years does not concur with facts. The Shanchol that is intended for field testing soon in Haiti had offered a poor protection of 45 percent during the first year of surveillance in a large-scale field trial in India in 2006. Dismal results were obtained in a large-scale field trial in Peru in 1994 when the two-dose vaccine Dukoral was tested.
Harmon has noted the approval of the oral cholera vaccines by the World Health Organization (WHO). A critical analysis of the WHO’s position on cholera vaccines is provided below, as transparency inside the WHO’s cholera vaccination program is badly needed. The WHO cholera vaccination program is controlled by a group of scientists who are linked with Shanchol and Dukoral. These scientists push their agenda through the WHO by spreading misleading information and advocating the use of these ineffective but commercially profitable vaccines.
None of these vaccines is suitable for controlling endemic or epidemic cholera. Vaccination can give people a false sense of security and cause them to become less cautious in matters related to hygiene. Consequently, an inefficient vaccine can even play a harmful role.
Instead of offering an useless and inefficient vaccine such as Shanchol to the population of Haiti, it would be better to provide more sources of potable water and more oral rehydration solution and treatment facilities to combat cholera. Such effective treatment can bring down mortality to less than one percent.
Download article PDF here or proceed to article below.
INTRODUCTION
In an article entitled “Can a Vaccine Cure Haiti’s Cholera” (Scientific American, January 12, 2012), Katherine Harmon focused on current discussions related to the application of killed oral cholera vaccines as a cholera-control measure in Haiti (1). Harmon presented the viewpoints of those who want to use oral cholera vaccines and those who are skeptical about their use for tackling the cholera that has affected the entire country. The information on the protective efficacies of two killed oral cholera vaccines, Shanchol and Dukoral, presented by Harmon is misleading. Shanchol and Dukoral contain large amounts of killed cholera bacteria, the later having an additional component known as the recombinant B subunit of cholera toxin (rCTB). Shanchol is intended for field testing soon on 100,000 inhabitants of Haiti.
Guidelines of the Office of Research Integrity (ORI) of the US Government
Before discussing the merits of these vaccines, it is essential to present the guidelines of ORI that strive to promote responsible conduct of research. ORI urges authors to maintain scientific integrity by
- (i) being accurate and honest,
- (ii) relying upon primary literature and
- (iii) avoiding the publication of selective information (2).
Harmon presented extensive information supplied by the members of Partners In Health (PIH), a Boston based organization that plans to work in Haiti on the forthcoming cholera vaccine trial.
According to Harmon, Dukoral and Shanchol offer 60 to 90 percent protection lasting for two to three years (1). But she failed to support this assertion with any primary source, thus violating the guidelines put forward by ORI.
Subsequently, she mentions Paul Farmer of PIH as stating that oral cholera vaccines are “80 percent effective”. But Paul Farmer also neglects to mention the primary source of this information. Paul Farmer is a Harvard professor in the Department of Global Health and Chief Strategist at PIH. However, he has ignored the directives of ORI that urge scientists to present their primary sources of information. It is not the only occasion on which he has done so.
In an article published in Newsweek (December 13, 2010) less than two months after the cholera outbreak, Farmer noted an even a higher number and said that the oral cholera vaccines were 90 percent protective (3). Farmer has not done any work on cholera vaccines and cannot be regarded as a reliable source of information on cholera vaccines.
Contrary to the claims of Katherine Harmon and Paul Farmer, Shanchol, which underwent a large-scale field trial in 2006 among the slum dwellers of Kolkata (India), offered only 45 percent protection during the first year of surveillance as reported in The Lancet (Reference 4, The Lancet, year 2009, volume 374, page 1699, Table 3).
Who are the cholera vaccine experts of the WHO?
As Katherine Harmon has written on the approval of oral cholera vaccines by the WHO, a detailed analysis of the WHO’s position on cholera vaccines is essential.
A critical examination of the composition of the members of the Cholera Vaccine Working Group who met on October 28, 2009 at the WHO Headquarter to recommend the use of the killed oral cholera vaccines would have revealed that most of the key players of this group had conflicts of interest, as they represented the interest of Dukoral and Shanchol, sold by private vaccine companies (4 -8).
Of 11 members of the group, 5 members (John Clemens, Ann-Mari Svennerholm , David Sack, Balakirish Nair, Dipika Sur) were actively associated with Dukoral and Shanchol. Five members (Zulfikar Bhutta, Anita Zaidi, Mitchell Weiss, Duncan Steele, Myriam Henekens) had made hardly any contribution in the field of cholera, their names appearing only sporadically in minor publications. Only one member (James Kaper) was a reputable scientist working with live oral cholera vaccines. Unfortunately, his vaccine (CVD 103-HgR) did not work when field tested in Indonesia in 1992 (9). There were a few other participants in that Working Group meeting. With the exception of one (Eric Mintz), most of them had contributed little to the field of cholera.
How were the members of the Working Group selected? One can demand transparency in the selection of the WHO’s cholera vaccine experts. Like the WHO’s swine flu experts, the vast majority of the WHO’s cholera experts are linked to the killed oral cholera vaccines and are pushing their agenda through the WHO by advocating the use of ineffective but commercially profitable vaccines such as Dukoral and Shanchol.
The WHO position paper on cholera vaccines: a catalogue of factual misrepresentations running contrary to ORI guidelines
The WHO has put forward misleading information in its position paper on cholera vaccines to promote the marketability of the killed oral cholera vaccines, Dukoral and Shanchol (10). Since Shanchol is meant to be field tested in Haiti soon, the information presented by the WHO on this vaccine in its position paper is examined first.
A. Shanchol
Shanchol is the trade name of an oral cholera vaccine comprising large amount of two groups of killed cholera bacteria (Vibrio cholerae O1 and O139). It is a two-dose vaccine to be taken orally with an interval of two weeks; immunity against cholera is expected to develop one week after the second dose (4).
A group of scientists from South Korea and Sweden (John Clemens, International Vaccine Institute and Jan Holmgren, Gothenburg, Sweden) are the prime architects of Shanchol (4). At first they worked on this vaccine in Vietnam (11). But the drug authority of Vietnam lacked international recognition. By contrast, the Central Drug Standard Control Organization of India was internationally recognized. To capture the global cholera vaccine market, they decided to work in India.
Shanchol is manufactured in India by Shantha Biotechnics, its parent company being the large French pharmaceutical company Sanofi-Aventis.
The prequalification of this vaccine by the WHO took place on September 29, 2011 (12).
In 2006, the vaccine was subjected to a large-scale field trial on the poor slum dwellers of Kolkata ( India) and the trial results during a surveillance period of two years were published in the Lancet (4).
The WHO concealed information on the efficacy, cost and composition of Shanchol
a. Shanchol’s very high bacterial content
Scientists associated with Shanchol, some of them belonging to the Cholera Vaccine Working Group of WHO, concealed information on the vaccine’s bacterial content in their publication in the Lancet (4). This information was hidden under an undefined term called ELISA UNIT and was not mentioned in the WHO’s position paper on cholera vaccines (10). However, the protocol of the Vaccine’s Kolkata trial of 2006 stated that a very large number of cholera bacteria (1.75 x 1011, or 175 billion) were present per dose (13). Since two doses are required for the immunization course, a recipient would get 22 times more bacteria in the immunization course than in a moderately effective injectable cholera vaccine used in the past (14).
b. Presence of mercury in Shanchol
The WHO position paper on cholera vaccines made no mention at all of the presence of the mercury containing compound thiomersal in Shanchol (10). To avoid any possible toxic side-effects, most of the vaccines produced in Europe and USA are free of thiomersal.
But thiomersal is present in Shanchol at a concentration of 0.02 percent (w/v), each dose (1.5 milliliters) containing 300 microgram of thiomersal. This amount is over 6 times the amount allowed by the WHO for thiomersal’s use in a multi-dose vial, the range being 10 to 50 microgram per dose (15).
It is worthwhile to point out that the WHO Expert Committee on Biological Standardization recommends the use of a non-mercury based preservative in oral cholera vaccines (16). Ironically, Shanchol’s manufacturer turned a blind eye to the WHO’s recommendation.
c. Selective reporting of the protective efficacy of Shanchol
In the position paper on cholera vaccines (10), the WHO selectively reported Shanchol’s protective efficacy as being 67% after two years, while concealing this vaccine’s poor protective efficacy of 45% after one year (4).
Children under 5 represent the group most vulnerable to cholera. Yet this study published in The Lancet did not produce any protective efficacy data in various age groups including children under 5 during the first year of surveillance (4).
Instead of waning, the protective efficacy of the vaccine is reported as increasing significantly during the second year. How was it possible? The authors of the trial results have not come up with any satisfactory explanation (4).
The credibility of Shanchol’s trial in India is questionable. As Shanchol demonstrated a poor efficacy of 45 percent during the first year of surveillance, it cannot be regarded as an effective vaccine for cholera control. Further, this type of selective reporting of scientific results is incompatible with the universally accepted guidelines of ethical writing endorsed by the ORI (2).
The performance of Shanchol was much poorer than that of the injectable cholera vaccine with adjuvant tested in the same Indian city of Kolkata in the 1970s (17). Shanchol’s protection rate of 45 percent during the first year of surveillance (4) is lower than that of an average injectable cholera vaccine, as estimated by Britain’s Cochrane group (18, 19).
Carriers play the most vital role in the dissemination of cholera in endemic communities and also help to perpetuate the infection. The effect of Shanchol in reducing the incidence of cholera carriers is unknown. Contrary to the claims of Jonathan Weigel of PHI (1), no evidence of herd immunity conferred by Shanchol to unvaccinated individuals in the area of vaccination has been demonstrated.
(d) Questionable information on cost of Shanchol
In its position paper on cholera vaccines, the WHO described Shanchol as a cheap vaccine that costs US $1.0 per dose (10). But the WHO does not mention how this cost was estimated. Two doses of Shanchol required for the immunization course contain large amount of bacteria, 22 times more than that present in a killed injectable cholera vaccine (14).
According to the reports in the Indian press (20), the commercially available two doses of Shanchol were priced at Indian rupees 600 (approximately US $12). Contrary to the WHO’s propaganda, a vaccine that costs US $12 is not cheap for cholera-afflicted poor people anywhere in the world. It is a bluff thrown by Shanchol’s prime pushers associated with the WHO.
They did this on a previous occasion with a killed oral cholera vaccine tested in Bangladesh in 1985, details of which have been described elsewhere (21). Now a modified and weaker version of that vaccine (Dukoral) is sold in Europe to rich tourists at an exorbitant price of US $75.
On the limitations of Shanchol for controlling cholera
As Shanchol is a spaced two-dose vaccine that is meant to confer immunity to the recipient one week after the last intake, it has questionable utility once a cholera epidemic has started (since a person could still get cholera after the first dose). Further, its poor efficacy soon after its intake makes it ineffective for cholera control. Besides Shanchol has a cold chain requirement, as the vaccine must be stored at 2-8 degree Celsius (refrigerated) (4). This is problematic, as many cholera-afflicted nations have tropical and subtropical climates and suffer from chronic shortages of electricity. In brief, Shanchol is unsuitable to control cholera effectively, whether the cholera is epidemic or endemic.
B. Dukoral
(a) False information provided on the field testing of Dukoral (WC-rCTB) in Bangladesh
Dukoral is a combination vaccine that contains the killed Vibrio cholerae O1 (both biotypes and serotypes) whole cell vaccine (WC) in combination with the recombinant cholera toxin B subunit (rCTB). Dukoral is currently sold by the Dutch vaccine company Crucell.
Two scientists from Sweden’s Gothenburg University (Jan Holmgren and his wife Ann-Mari Svennerholm) have been described as the inventors of Dukoral (22). Incidentally, both of them are long-time associates of the WHO’s cholera program, Ann-Mari Svennerholm attending the WHO’s Cholera Vaccine Group meeting on October 28, 2009 as a full member (6).
For years, the WHO has falsely reported that the Dukoral (WC-rCTB) vaccine was field tested in Bangladesh (23-25). The WHO has never produced any primary references to support this claim. No vaccine containing WC-rCTB was ever subjected to a large-scale field trial in Bangladesh. Since the production of rCTB by recombinant DNA technology was only first reported in 1989, its use in the oral cholera vaccine could not have started until the 1990s (26, 27).
What was tested in a large-scale field trial in Bangladesh in 1985 was a WC-CTB vaccine containing a large number of killed whole cells (WC) in combination with a cholera toxin B subunit (CTB) that was not produced by recombinant methods but had been isolated chemically from cell-free culture supernatants [28]. [Such preparations contain impurities that would not occur in the rCTB preparations. See below.]
The recombinant version, rCTB, was not available at that time and hence no Dukoral (by defitition, WC-rCTB) was field tested in Bangladesh in 1985. As accuracy of facts must be maintained, the results from the 1985 Bangladeshi trial of oral cholera vaccine cannot be manipulated and falsified by stating that those results were obtained using Dukoral. Any attempt to describe the field testing of Dukoral in Bangladesh in 1985 constitutes acts of fabrication and falsification, which run contrary to the guidelines of ethically appropriate writing practices put forward by the Office of Research Integrity (ORI) of the US Government (2).
The isolation of rCTB by recombinant DNA technology involves procedures that do not encounter cholera toxin (CT). Moreover, when CTB is prepared from different sources the results are not necessarily be the same. CTB obtained by chemical purification of the cell free supernatant may contain traces of cholera toxin (CT), a very powerful adjuvant (29), that may remain undetected by the quality control assay but be sufficient enough to exert some of its biological functions. The substitution of CTB by rCTB can weaken the combination vaccine. Other reports have correctly distinguished between WC-CTB and WC-rCTB (30, 31).
Although tests on a few Swedish volunteers showed that their serum had more antitoxin and vibriocidal antibody after oral immunization with WC-rCTB or WC-CTB, the study did not involve challenging the volunteers with live Vibrio cholerae O1 to test their immunity; therefore no information was provided by this study on the comparative protective efficacies of these two vaccines [32, 33]. The purity of chemically isolated products can vary from lot to lot. The WC-CTB vaccine used in the Swedish study was not from the same lot as the one used in the Bangladeshi trial of 1985 (32).
It is worthwhile to point out that no field trial simultaneously comparing the protective efficacies of WC-CTB and WC-rCTB has ever been carried out. Therefore, the “practical” identity between CTB and rCTB, as mentioned in the position paper on cholera vaccines put forward by the WHO, is not supported by adequate evidence (10).
In several places in the position paper on cholera vaccines (10), the WHO falsely claimed that the data generated by the WC-CTB vaccine were the same as for the Dukoral, which contains WC-rCTB (instead of WC-CTB).
This is not the only case where false and fabricated information appeared in the Weekly Epidemiological Record (WER). In a publication in 2004, WER stated that 2 doses of WC-rCTB were given to trial participants in Bangladesh (23). This statement was false, since participants in the Bangladesh 1985 field trial were fed 3 spaced doses of the WC-CTB vaccine (28).
No WC-rCTB was field ever tested in Bangladesh, as WC-rCTB did not exist in the 1980s.
Further, the WER’s claim, in a number of publications for a number of years, that WC-rCTB was subjected to a field trial in Sweden is false (24, 25), as no field trial of an oral cholera vaccine was ever carried out in Sweden. Testing for immunogenicity of the vaccine, without any challenge study with live Vibrio cholerae O1, on a handful of healthy volunteers of a non-endemic country, does not constitute a field trial of a vaccine.
(b) Dukoral offered poor protection against cholera in large-scale field trial
The results of field trials of Dukoral have varied, dependent on who conducted them: scientists associated with Dukoral, versus others with more independent scientific judgment.
A small-scale trial of Dukoral carried out for a period of 18 weeks on 1426 military recruits of Peru in 1994 had offered protective efficacy of 86 percent against cholera (27). The relatively “high rate of protection” was due to very few instances of cholera, in any case, and a reassignment of the military recruits to other bases, which led to the early closure of the trial (34).
A large-scale field trial of Dukoral was conducted in Peru in 1994 by a group of American scientists who were independent of the developers of Dukoral. Dismal results were obtained when 10 592 persons were vaccinated with two doses of the Dukoral (35).
The Peruvian study failed to detect any protection after 1 year in children below 5 years and found very little protection (only 15 percent) in the age group above 5 years (35). Protection level during the 2nd year of surveillance increased only when a third booster dose was administered 10 months after the second one, protection being 61% for all age groups and 50% for children (2-15 years). The trial was not monitored beyond the 2nd year.
However, when John Clemens, a key player behind Dukoral, tested 2-doses of Dukoral in Mozambique in 2004, the vaccine had offered 82 and 67 percent protection respectively for below 5 and above 5 years of age respectively (36). This trial was conducted only for a period of 6 months, too short for adequate measurement of the protective efficacy of the Dukoral.
(c ) Selective reporting on the field trials of Dukoral (WC-rCTB) in Peru
After the publication of the results of the large scale field trial in Peru, a debate took place in the Journal of Infectious Diseases where the viewpoints of those supporting Dukoral’s 2-dose regimen (37) were rebutted by the scientists who conducted the trial (34).
In the position paper on cholera vaccines (10), the WHO selectively cited only the remarks of associates of Dukoral (John Clemens and David Sack, who were also members of the Cholera Vaccine Working Group of October 28, 2009) on the Peruvian trial of WC-rCTB, while concealing the rebuttal of those who had conducted the trial (34).
According to the proponents (John Clemens and David Sack) of the 2-dose regimen, 2 doses of the WC-CTB vaccine were as effective as 3 doses, as found in Bangladesh in the 1980s [37, 38]. However, a recent study on oral cholera vaccines, initiated by Britain’s Cochrane Infectious Diseases Group, has “been unable to get access to the data to confirm this finding” arising out of Bangladesh (39).
On the limitation of Dukoral in controlling cholera
Several factors limit the applicability of Dukoral as a cholera control measure in epidemics. Since 2 doses of these vaccines are administered with an interval of 2 weeks, protective efficacy starts one week after the final dose, making these vaccines of little value once a cholera outbreak has started. Besides, one can get cholera during the interval between two doses. This is why Dukoral was not used in recent cholera outbreaks in Iraq and Zimbabwe (40, 41).
Dukoral requires large quantity of safe water as the product is very voluminous, 30 times more than other usual vaccines (40). Dukoral cannot be given to children under the age of 2 (35). The vaccine delivery system is inconvenient, requiring stomach acid neutralization, which can be problematic for people with stomach ailments.
The vaccine’s short-term protective efficacy, poor protection in younger children, strict requirement of cold chain and very high cost make Dukoral unsuitable for use in cholera endemic countries (31, 38, 40-44).
CONCLUDING REMARKS
Neither Shanchol nor Dukoral is suitable for controlling cholera effectively, whether the cholera is epidemic or endemic.
- Katherine Harmon’s statement regarding these vaccines’ efficacy of 60 to 90 percent over a period of two to three years is not supported by scientific evidence.
- Paul Farmer, not an authority on cholera vaccines, has exaggerated the protective efficacies of these vaccines.
- Shanchol that is meant to be field tested in Haiti soon had offered only 45 percent protection during the first year of surveillance in a large scale field trial in India (4).
- Dukoral, the two-dose cholera vaccine, had offered dismal results in a large scale field trial in Peru conducted by a group of investigators independent of Dukoral’s developers.
- No Dukoral was ever subjected to a large scale field trial in Bangladesh.
- No field trial data of Dukoral lasting for a period of two to three years exist.
As scientists associated with Shanchol and Dukoral control the WHO’s cholera vaccination program, they have used the umbrella of the WHO to spread false and misleading information on these vaccines (10).
Although cholera can be life-threatening, it is an easily treatable disease that can be effectively cured by different rehydration therapy (oral or intravenous fluids) and if necessary, by antibiotics (45). To develop an effective single dose vaccine that can provide long lasting immunity against cholera, further research would be needed.
The waxing and waning of outbreaks is a familiar picture in cholera epidemics (46). In 2009, Zimbabwe had encountered a cholera outbreak that killed more than 4 000 people (47). But in the following year there were very few cases of cholera.
Skepticism of David Olson, a medical advisor of diarrhoeal diseases working for “Doctors Without Borders” and Andrea Vicari, a vaccination advisor for the Pan-American Health Organization (PAHO) on the cholera vaccination program in Haiti, as reported in Katherine Harmon’s article, is worth noting (1). In a country with a population of approximately 10 million, one has
“to consider nearly everybody at risk in Haiti for cholera….. To vaccinate the entire country with the required two-doses per person, it would … take a five-year effort (with immunity waning in each person after two years)..” (1).
Use of inefficient and expensive vaccines such as Shanchol and Dukoral has little justification in controlling cholera in Haiti. Besides, vaccination can give people a false sense of security and may induce them to be less careful in handling food and drinks. In this respect, an inefficient vaccine can even cause harm.
Articles dealing with cholera vaccines should provide information which is adequate, accurate, and based on facts. Inaccurate information, as presented in the article of Katherine Harmon, runs contrary to the accepted norms of ethical writing practices outlined by the guidelines of ORI (2). This may mislead readers, including those dealing with public health policy matters, with detrimental consequences on the welfare of populations vulnerable to cholera.
REFERENCES
1. Harmon K. Can a Vaccine Cure Haiti’s Cholera? Scientific American, January 12, 2012
2. Office of Research Integrity. US Department of Health and Human Services.
Avoiding plagiarism, self-plagiarism, and other questionable writing practices: A guide
to ethical writing. http://ori.hhs.gov/education/products/plagiarism/1.shtml
3. Farmer P., Rejouit JR. How we can stop cholera. Newsweek, December 13, 2010
4. Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. Efficacy and safety of a modified killed-whole cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009, 374(9702):1694-702.
5. Weekly Epidemiological Record, Meeting of the Strategic Advisory Group of
Experts on immunization, October 2009 – conclusions and recommendations.
No. 50, 2009, 84, 517-532.
6. http://www.who.int/immunization/sage/SAGE_cholera_overview_slides_10-28-09_Jon_Abramson.pdf
7. Sanchez JL, Trofa AF, Taylor DN, Kuschner RA, DeFraites RF, Craig SC, Rao MR, Clemens JD, Svennerholm AM, Sadoff JC, et al. Safety and immunogenicity of the oral, whole cell/recombinant B subunit cholera vaccine in North American volunteers.
J Infect Dis. 1993;167:1446-9.
8. Clemens JD, Harris JR, Khan MR, Ali M, Younus M, Khan MU et al., Impact of B subunit killed the whole-cell and killed whole-cell-only oral vaccines against cholera upon treated diarrhoeal illness and mortality in an area endemic for cholera. Lancet 1988 Jun 18: 1375-79.
9. Richie EE, Punjabi NH, Sidharta YY, Peetosutan KK, Sukandar MM, Wasserman SS, Lesmana MM, Wangsasaputra FF, Pandam SS, Levine MM, O’Hanley PP, Cryz SJ, Simanjuntak CH. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000, 18:2399-410.
10. Cholera vaccines: WHO position paper, Weekly Epidemiological Report, No 13, 2010, 85, 117-128.
11. Thiem VD, Deen JL, von Seidlein L, Canh do G, Anh DD, Park JK, Ali M, Danovaro-Holliday MC, Son ND, Hoa NT, Holmgren J, Clemens JD. Long-term effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine. 2006; 24:4297-303.
12. www.who.int/immunization_standards/vaccine_quality/pq_250_cholera_1dose_shantha/en/
13. A Radomized Controlled Trial of the Bivalent Killed Whole Cell Oral Cholera Vaccine in Eastern Kolkata, West Bengal, India. Protocol/Version Number C-8-Pill version 3.0 Section 6.1 Study agents (Vaccine and placebo)
14. Mosley WH., McCormack WM, Fahimuddin M, Aziz KMA, Rahman ASMM et al.,
Report of the 1966-67 cholera vaccine field trial in rural East Pakistan. Bull World Health Orgn. 1969, 40, 177-185.
15. WHO. 2006. Global Advisory Committee on Vaccine safety , Thiomersal and vaccines: questions and answers. http://www.who.int/vaccine_safety/topics/thiomersal/questions/en/
16. WHO Expert Committee on Biological standardization, Technical Report Series No 2004; 924: 1-232.
17. Pal SC, Deb BC, Sen Gupta PG, De SP, Sircar BK, Sen D, Sikdar SN. A controlled field trial of an aluminum phosphate-adsorbed cholera vaccine in Calcutta. Bull World Health Organ. 1980; 58:741-5.
18. Graves P, Deeks J, Demicheli, V, Pratt M, Jefferson T. Vaccines for preventing cholera. Cochrane Database Syst Rev 2000; 4: CD000974.
19. Jefferson T. What are the benefits of editorials and non-systematic review? The British Medical Journal 199; 318:135 (9 January).
20. Business Daily from The Hindu group of publications, Oral cholera vaccine Shanchol from Shantha for India, 27 April 2009.
21. Ahmed S. Marketing of the Oral Cholera Vaccine Dukoral using Misleading Information and the Exploitation of Women and Children of Bangladesh as Experimental Animals. News From Bangladesh. January 24, 2011 (http://bangladesh-web.com/view.php?hidRecord=346593 )
22. DUKORAL: Resevacciner från SBL Vaccin, 105 21 Stockholm, Sweden.
23. Cholera 2003. Weekly Epidemiological Record, No 31, 2004, 79, 281-288.
24. Cholera 2002. Weekly Epidemiological Record, No 31, 2003, 78, 269-276.
25. Cholera 2001. Weekly Epidemiological Record, No 31, 2002, 77, 257-268.
26. Sanchez J, Holmgren J. 1989. Recombinant system for over expression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proceedings of the National Academy of Sciences U S A. 86(2):481-5.
27. Sanchez JL, Vasquez B, Begue RE, Meza R, Castellares G, et al., Protective
efficacy of oral the whole-cell/recombinant-B-subunit cholera vaccine in Peruvian
military recruits. Lancet. 1994; 344(8932):1273-6.
28. Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, Kay BA, Khan MU, Yunus M, Atkinson W, et al. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986, 2(8499):124-7.
29. Northrup RS, Fauci AS. Adjuvant effect of cholera enterotoxin on the immune
response of the mouse to sheep red blood cells. J Infect Dis 1972; 125:672-3.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines: use in clinical practice. Lancet
Infect Dis 2006; 6:361-73.
31. Kabir S. Cholera vaccines: current status and problems. Rev Med Microbiol 2005; 16:101-16.
32. Jertborn M, Svennerholm AM, Holmgren J. Safety and immunogenicity of an oral
recombinant cholera B subunit-the whole cell vaccine in Swedish volunteers.
Vaccine. 1992;10:130-2.
33. Jertborn M, Svennerholm AM, Holmgren J. Immunological memory after immunization with oral cholera B subunit—the whole-cell vaccine in Swedish volunteers. Vaccine. 1994;12:1078-82.
34. Taylor DN, Sanchez J, Cardenas V, Gilman RE, Sadoff, J. Reply to misleading
negative findings in a field trial of killed, oral cholera vaccine in Peru. J Infect Dis.
2001; 183: 1308-9.
35. Taylor DN, Cárdenas V, Sanchez JL, Bégué RE, Gilman R. Two-year study of the
protective efficacy of the oral whole cell plus recombinant B subunit cholera vaccine in Peru. J Infect Dis. 2000;181: 1667-73.
36. Lucas ME, Deen JL, von Seidlein L, Wang XY, Ampuero J. et al., Effectiveness of
mass oral cholera vaccination in Beira, Mozambique. N Engl J Med. 2005; 352: 757-67.
37. Clemens JD, Sack DA, Ivanoff B. Misleading negative findings in a field trial of
killed, oral cholera vaccine in Peru. J Infect Dis. 2001; 183:1306-8.
38. Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270-3.
39. Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. Oral vaccines for preventing
cholera. Cochrane Database Syst Rev. 2011;(3):CD008603.
40. The WHO position paper on cholera vaccine use in Iraq, 05 October 2007
41. The WHO, Global Alert and Response, Cholera in Zimbabwe, December 2, 2008; http://www.The WHO.int/csr/don/2008_12_02/en/index.html
42. Kabir S. Cholera vaccines. Lancet Infect Dis. 2007; 7:176-8.
43. Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, Khan MU, Kay BA, Huda N, Khan MR, et al. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988 Jul;158(1):60-9.
44. Finkelstein RA. Why do we not yet have a suitable vaccine against cholera? Adv Exp Med Biol. 1995;371B:1633-40. Review.
45. WHO. 2004. Cholera Outbreak, Assessing the outbreak response and improving
preparedness. pages 1-90.
46. Editorial, The Lancet Infectious Diseases 10(12), 813, December 2010.
47. Zimbabwe: cholera keeps a low profile
http://www.irinnews.org/Report.aspx?ReportId=87828
Humanitarian news and analysis: a service of the UN Office for the Coordination of
Humanitarian Affairs. January 21, 2010
Source: Haiti Chery

![Panel of Experts[7]](http://www.dadychery.org/wp-content/uploads/2012/01/Panel-of-Experts7.jpg)


Comments
Oral Cholera Vaccines Cannot Control Haiti Cholera: Rebuttal to an Article in Scientific American — No Comments